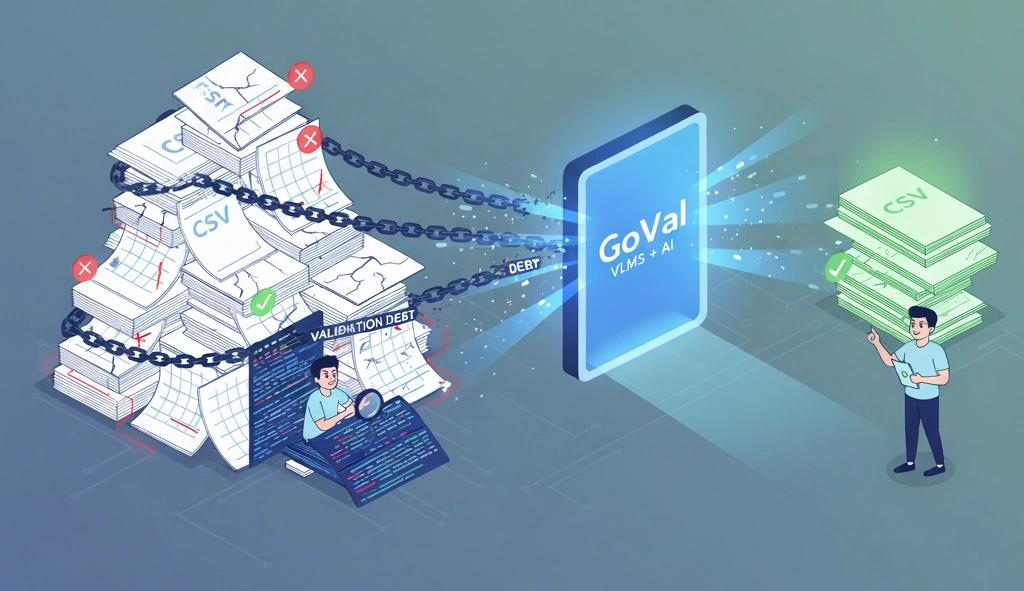
A Unified Approach to GxP Validation: Biotech, Medical Devices & Clinical Trials
Master GxP compliance across diverse Life Sciences sectors. Learn how a unified validation platform streamlines biotechnology validation, medical device validation, and clinical trial validation with tailored, risk-based workflows.
Read More