Ensuring 21 CFR Part 11 Compliance with Electronic Records, Electronic Signatures, and Audit Trails
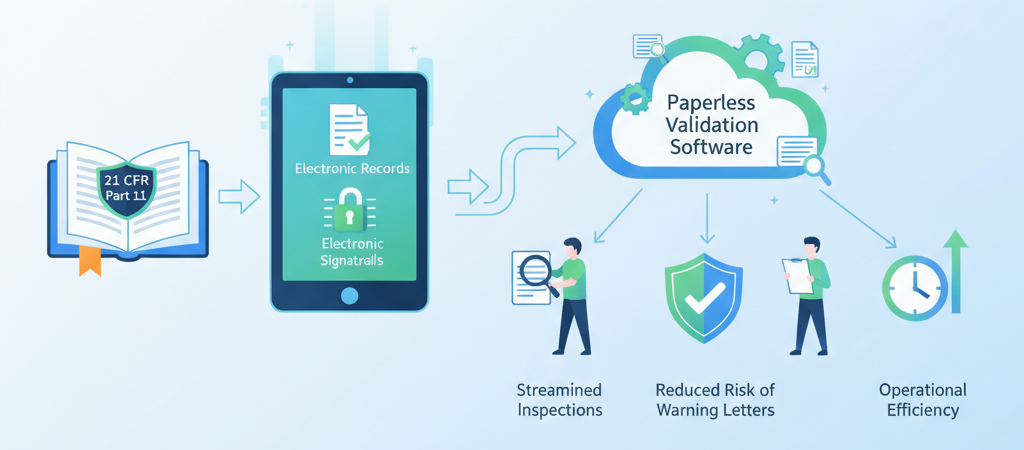
In the regulated landscape of pharmaceutical and life sciences, U.S. FDA’s 21 CFR Part 11 sets rigorous standards for electronic records and electronic signatures. Meeting these requirements not only avoids warning letters but also establishes an audit-ready culture that strengthens data integrity, transparency, and operational efficiency.
Why Electronic Records and Electronic Signatures Matter
- Data Integrity and Authenticity Electronic records, when managed correctly, are tamper-evident and time-stamped. Every modification is logged, ensuring that the data you present during an FDA inspection is trustworthy and unaltered.
- Accountability Through Electronic Signatures Part 11 requires that electronic signatures be uniquely linked to their signers and that their identity can be verified. This ensures that every approval or document is traceable to a specific individual, reinforcing personal accountability.
- Comprehensive Audit Trails Audit trails automatically record who did what and when—including record creation, edits, reviews, and sign-offs. During an FDA audit, this detailed history demonstrates continuous compliance and deters unauthorized changes.
Key Benefits for Audit-Ready Operations
- Streamlined Inspections With complete e-records and audit logs, inspectors can immediately verify compliance; no more scrambling for paper files or reconstructing missing signatures.
- Reduced Risk of Warning Letters Thorough documentation of compliance controls—electronic signatures, user authentication, role-based access—minimizes the chance of FDA citations for inadequate record keeping.
- Operational Efficiency Transitioning from paper to digital records reduces manual handling errors, accelerates approval cycles, and frees staff from tedious filing tasks.
Critical Features of a Part 11-Compliant Software Solution
- Paperless Validation Eliminate stacks of binders and paper forms. A digital validation library stores all documentation—URS, test protocols, summary reports—in one secure repository accessible anywhere.
- Template-Based Validation Documents Ready-made templates for protocols, deviation reports, and traceability matrices ensure consistency and adherence to GxP guidelines while slashing document preparation time.
- Configurable Workflows Define electronic “to-do” pipelines for draft review, stakeholder approvals, and final sign-off. Automated notifications keep validation projects on schedule and visible to all team members.
- User-centric Access Controls Enforce stringent role-based permissions so only authorized personnel can view, edit, or sign off on specific validation records.
How GoVal Empowers 21 CFR Part 11 Compliance
GoVal’s Validation Lifecycle Management System is architected to satisfy every aspect of Part 11:
- Secure Electronic Records All records are encrypted at rest and in transit. Immutable audit trails capture every user action, complete with timestamps and IP addresses.
- Robust Electronic Signatures GoVal ties each signature to multi-factor authenticated user accounts. Sign-offs automatically append the signer’s credentials and rationale.
- Audit-Ready Dashboards A consolidated overview displays compliance status, open tasks, and audit logs in real time—eliminating surprises and ensuring inspectors see a well-organized system.
- Paperless Validation Library Store and retrieve validation deliverables instantly. Digital archiving means no lost files and instant retrieval for audits or management reviews.
- Template-Driven Documents Out-of-the-box templates cover URS, IQ/OQ/PQ, test protocols, deviation reports, and summary reports. Customization enables alignment with internal SOPs.
- Automated Workflows Configure branching workflows to route documents for review, approval, and signature. Automated reminders and escalation rules ensure timely completion.
- Reporting and Analytics Generate compliance metrics, trend analyses, and executive summaries with a few clicks—delivering actionable insights and transparency for management and regulators.
Conclusion
Adopting a Part 11-compliant digital validation platform transforms your organization’s approach to regulatory compliance. Electronic records, signatures, and audit trails create an unbroken chain of trust in your data—dramatically reducing risks of FDA warning letters and elevating your audit readiness. With GoVal’s paperless, template-based, workflow-driven solution, achieving and maintaining 21 CFR Part 11 compliance becomes a streamlined, transparent, and efficient process.